Our Plant Tour
Our State of Art Facility
Our State of Art Facility offers successful marketing operations for the Exports market. It was a matter of backward integration for VIDHYASHA to set up a state of the art manufacturing facility at Kala amb, H.P. to ensure prompt supply of top quality products. This facility has been certified asWHO-GMP & ISO 9001: 2008, ISO 22000:2005 company.. Located at Dist. Sirmour, H.P. at the foot hills of Shivalik range in North India, this manufacturing facility is built with ultra modern facilities.
.
The current facility has a capacity to manufacture 925 million tablets , 220 million softgels, & 110 million hard gelatin capsules per annum.

Quality Assurance, Quality Control, Instrumentation Area
The Quality Assurance (QA) system is an integral part of a healthcare product manufacturer. The role of the Quality Assurance is to co-ordinate the development and maintenance of the company’s Quality procedures and systems. There are standard operating procedures (SOP’s) for all activities including Raw material procurement, Production, Quality Control, Material Management, Engineering, Environmental Controls, House Keeping and Sanitization. Quality Assurance ensures that these procedures are adhered to and the records of the same are maintained. QA ensures that necessary measures and relevant tests are undertaken before the products are released for use or supply. A major portion of the analytical testing done on the raw materials and finished products is accomplished in-house and is carried out by qualified staff. The analytical processes are under the control of qualified technical staff. The Quality Control laboratory is equipped with analytical instruments such as HPLC (brands complying with the cGMP and CFR requirements), UV Spectrophotometer, Dissolution Apparatus, FTIR et al. The microbiology department is equipped to carry out the necessary microbial tests that are an integral part for products involving the use of gelatin. The microbiology department is equipped with double door high pressure steam sterilizer (Autoclave), BOD incubators, LAF and a TOC analyzer to assure the highest quality of water. Quality is mandated by the management and assured by the technical staff at every level of operation. The effectiveness and applicability of Quality Assurance is regularly monitored through an internal audit system at regular intervals. Water System Considering the criticality of the water quality the facility is equipped with a State of the art Water system meeting USP guidelines.

Water System
Considering the criticality of the water quality the facility is equipped with a State of the Water system meeting USP guidelines. The present water system comprises of a Double Pass Reverse Osmosis treatment of water that is followed Electro-deionization treatment to produce purified water that complies with the USP requirements.
This system is equipped with on-line sensors that record the pH and conductivity parameters and they are continuously monitored round the clock via a synchronized PLC system. It is a fully automated water system that is controlled via SCADA (Supervisory Control and Data Acquisition System).
Purified water is stored in insulated storage tanks and supplied to different user points via a continuous recirculation loop.

Packaging
Vidhyasha’s experienced quality control department maintains the integrity, honesty, and reliability of our spectrum of products and extends to swathe the packaging of every product to maintain safety and stability. In every packaging option, packaging materials and processes confirm to international standards, with regard to a host of critical parameters, including :

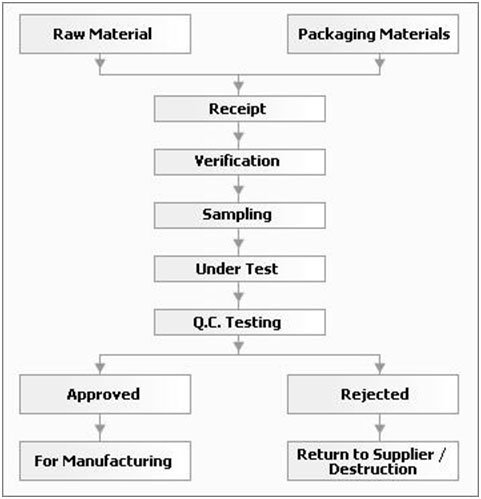
RM STORE PROCESS
SOFT GELATIN CAPSULE MANUFACTURING

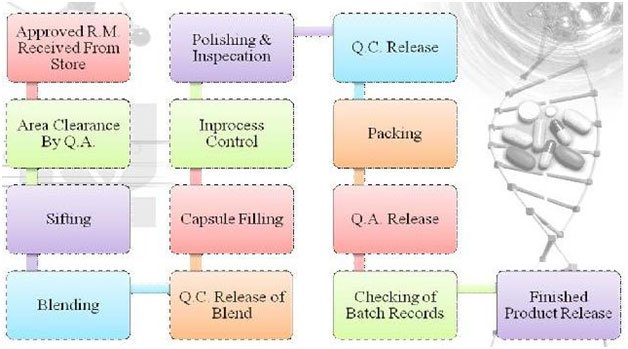
HARD GELATIN CAPSULE MANUFACTURING